From engineering applications to construction and everyday chemicals, nylon continues to be one of the most prominent and extensively employed engineering plastics. Though not every nylon is the same. The most well-known types are Nylon PA6 and PA66. Each has its own distinctive traits, advantages, and uses. But how do you determine which one perfectly fits your requirements? This article focuses on the primary distinctions between these two products and their material properties, condition-specific performance, and typical uses. Be it ultra-precise parts design and materials selection for production, or simply wondering how these polymers work, you will find what will enable an educated decision. Continue reading so that you learn how these two nylons compare and where they stand apart.
What is the chemical structure of PA6 and PA66?
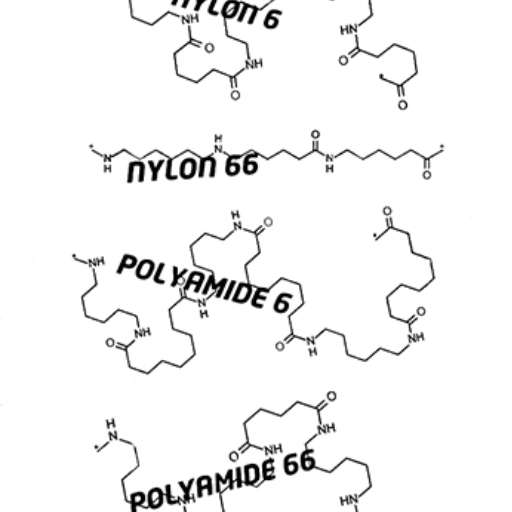
Both nylon PA6 and PA66 have unique differences in their chemical structure:
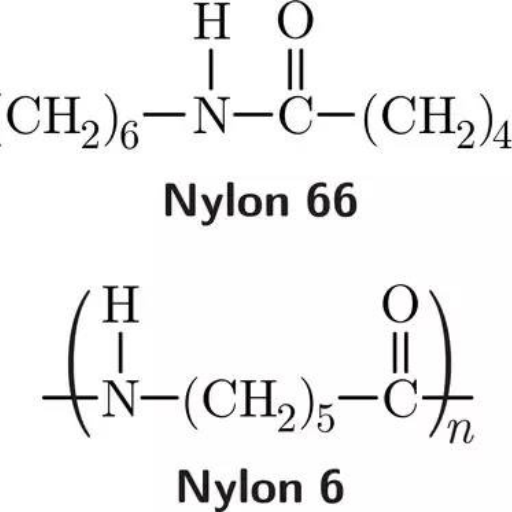
- PA6 (Polyamide 6): PA6 is generated by the polymerization of caprolactam, a monomer with a single caprolactam unit (C₆). Its skeletal structure includes polymerized contour unit chains with lines constituting fragments that are formed from this monomer.
- PA66 (Polyamide 66): It is manufactured through the polymerization of two monomers: hexamethylenediamine and adipic acid. Each contains six carbon atoms, thus creating a structure that includes repeating units of the two monomers.’
The main difference rests in the different material properties of each item which is determined by the amount and order of repeating units in the polymer chains.
How does the chemical structure of PA6 affect its properties?
The properties of PA6 plastics are greatly influenced by their monomer, methyl caprolactam, which is linked through amide bonds. PA6 is composed of repeating units of polyamide chains with hydrogen bonds, resulting in high tensile strength. The substantial molecular interactions in PA6 contribute to its high endurance toward abrasion, making it useful in mechanical and industrial hardware, including gears and bearings.
The value of PA6 lies in its semi-crystalline structure, which provides an unrivaled combination of toughness and flexibility. Compared to other engineering thermoplastics, PA6 has good impact resistance and superior resistance to chemicals such as oils and hydrocarbons, thanks to the tight binding of its molecular chains.
The thermal properties of PA6 are equally impressive, with a melting point of about 220 degrees Celsius and a continuous use temperature range of -40 to 120 degrees Celsius. This variation in temperature ranges makes PA6 a widely used material in the electrical and automobile industries.
Of note is that PA6 is hydrophilic, which means it readily absorbs moisture from the surrounding environment. This, in turn, affects its mechanical performance because water, when absorbed, acts as a plasticizer which tends to reduce stiffness and increase pliability. Water absorption also changes the geometry or shape of PA6, which absorbs water and transforms its geometric stability over a period of time; this so called ‘dimensional stability’ pliability of PA6 adds complexities that must be viewed during the design and production stages.
Gaining knowledge with PA6’s chemical and physical properties tells us why this Polyamide is still a preffereded option for different industrial activities.
What are the key differences in the chemical structure of PA6 and PA66?
The structural differences between PA6 (Polyamide 6) and PA66 (Polyamide 66) lead to differences in their physical and mechanical properties. While PA6 was synthesized only using caprolactam as a monomer and polymerization to a caprolactam homopolymer, PA66 is produced from the polymerization of a combination of two monomers, hexamethylenediamine and adipic acid, resulting in an alternating copolymer.
The repeating units possess a different number of carbon atoms for both compounds, hence constituting the primary difference in chemical structures. PA6 possesses 6 carbon atoms from caprolactam’s single monomer, whereas PA66 is composed of 6 carbon atoms from both diamine and diacid. The resulting carbon chain length variance directly influences the material’s density, crystallinity, and hydrogen bonding. PA66 tends to have a higher melting point (around 255°C) compared to PA6 (220°C) because of stronger structural organization and greater interchain hydrogen bonding. This results in superior mechanical strength, abrasion resistance, and high performance capabilities due to PA66’s heightened crystallinity.
The variations in structure impact water absorption behavior; for instance, PA6 tends to absorb more moisture due to its lesser degree of crystallinity, albeit both materials are hygroscopic. These differences matter in making the right choice for the use of PA6 and PA66 in particular engineering and industrial applications.
Why is polyamide 66 considered stronger?
Polyamide 66 (PA66) is stronger than polyamide 6 (PA6) because of its higher crystallinity. The increased crystallinity of PA66 allows for greater tensile strength, stiffness, and heat resistance, which makes it ideal in demanding applications. PA66’s mechanical robustness is reflected in its tensile strength, which is approximately 80 MPa, whilst PA6 is at 70 MPa.
Moreover, polyamide 66’s melting temperature of approximately 255°C makes it stronger than PA6’s 220°C. This assists in high temperature environments such as in vehicles, electrical components, and industrial machinery where structural integrity must be maintained. It also has better wear resistance and lower creep which assists the longevity of the components under mechanical stress.
Provided moisture absorption is managed, balanced parameters make PA66 more beneficial than PA6 in sectors requiring reliable strength and endurance in harsh conditions, making it appealing in industries where dependability under stress and heat is crucial.
What are the mechanical properties of PA6 and PA66?

Mechanical Properties of PA6 and PA66
- PA6 (Polyamide 6):
- Tensile Strength: Moderate
- Impact Resistance: Good
- Flexibility: Greater than PA66
- Moisture Absorption: Higher, may impair performance
- PA66 (Polyamide 66):
- Tensile Strength: High
- Impact Resistance: Moderate,
- Flexibility: Lower than PA6
-
Heat Resistance: Greater than PA6
How do the mechanical properties compare between PA6 and PA66?
In my view, PA6 is more flexible and has better impact resistance than PA66, but it absorbs more moisture, which may be detrimental to its performance. Conversely, PA66 is more suitable for highly demanding applications that require strength and thermal resistance due to its greater heat withstanding tensile strength.
What gives PA66 a higher melting point than PA6?
Having a schematic contour that integrates into the composition chain of PA66 as “more symmetric,c tightly packed polymer chain”, PA66 contains hexamethylenediamine and adipic acid, while PA6 is simple PA6.
Due to the structure, methanonic modifications of the polymer have stronger thermal intermolecular hydrogen bonding, which increases thermal stability and, as a result, increases the melting point of PA66 as compared to PA6, reason why PA66 has a melting point of 260 degrees centigrade while PA6 has 220. PA66 is more efficient thermally and less prone to thermal degradation, while PA6 is vulnerable to corrosion, making it less durable for heavy industrial machinery and engine components for automotive engines. These applications warrant for such materials highest thermal performance. PA66 is PA6 with a higher crystalline structure and, under physical strain, enables more rigidity.)
How does impact resistance differ in PA6 vs PA66?
The primary factors differentiating the impact resistance of PA6 and PA66 are their molecular structure and crystalline features. The greater flexibility and toughness associated with PA6 increases its impact resistance relative to PA66. In terms of lower course energy impact, PA6 dissipates energy better than PA66 with shock resistance which makes it favorable to applications such as safety parts or structural components which undergo dynamic loads.
PA66, on the other hand, exhibits greater rigidity and strength owing to higher crystallinity, thus leading to increased static load-bearing ability or continuous stress, but reduced impact resistance. This means that PA66 is able to withstand higher static forces, but is not as effective in the field where sudden shock impact response is necessary. For example, while PA66 outperforms under high temperature conditions, it lacks energy absorption under abrupt impacts, unlike PA6.
Material data indicates that the Izod impact strength of PA6 is 10-13 kJ/m² while that of PA66 is 7-10 kJ/m². From these figures, one can note the significant difference in the capabilities of PA6 and PA66, as the former can endure greater energy input during impact without fracturing as opposed to the latter.
How do PA6 and PA66 perform in temperature and electrical resistance?

PA6 and PA66 differ in terms of temperature and electrical resistance performance. PA66 typically has a higher melting temperature of around 255°C, in contrast to 220°C for PA6, thus making PA66 better suited for use in higher temperature applications. That said, both materials are able to withstand moderate temperatures.
In the context of electrical resistance, PA66 is superior due to possessing better dielectric strength and insulation properties than PA6, thus making it a better choice for use in electrical and electronics applications where insulation is paramount.
What are the thermal properties of PA6 compared to PA66?
Polyamide 6 (PA6) and Polyamide 66 (PA66) are examples of engineering plastics with useful applications, yet their differences in thermal properties, PA6’s lower melting point of about 220°C as opposed to PA66’s roughly 255°C, mean PA66 is preferred for hotter environments. The higher melting point of PA66 makes it a more suitable choice for long-term use in high-temperature environments like the automotive industry and industrial appliances.
Furthermore, PA66 has better deflection temperature (HDT) under load, meaning that it can sustain higher temperatures without losing structural strength. For example, PA66 has an HDT of 85°C to 100°C while PA6 has around 75°C-85°C, lower than PA66 but varying depending on the specific grade and reinforcements used.
Both materials have moderate endurance against rising temperatures, but PA66 and PA6’s preference is clear: while PA6 is suitable for environments without demanding conditions, PA66 is the ideal choice when facing the critical need for thermal endurance.
What makes PA66 have better electrical insulation properties?
The molecular structure of PA66, along with its material attributes, is the reason for its superior electrical insulation properties. Crystallinity of the Polymer AA66 Is PA66 Insulation PA 66 is5500.05 Also part crystalline single PA66 has it 3 also contributes to lowering the dielectric Polar strength and thus PA66 Insulation increases The effectiveness rate of PA66 is roughly 20-30 kV/mm. Depending on the grade and specimen, these ranges may vary due to the molecular composition. Besides these traits, PA66 boasts low moisture absorption and loss percentages increases its insulation. PA66 possesses loss comparison tracking index CTI values above PA6,6, indicating lesser electrical attacks to insulation damage. These characteristics deem it fit to be used with electrical connectors, switchgears, and other high-voltage appliances.
What are the applications of nylon PA6 and PA66 in industries?

The mechanical resistance, durability, and longevity of Nylon PA6 and PA66 make them popular choices across the board.
- Automotive Industry: The high performance of these materials, as well as their resistance to heat, makes them useful in the manufacture of engine parts, gears, and even interior furnishings.
- Electrical and Electronics: PA6 and PA66 Nylons are widely used in connectors of various types, including electric and switchgear, as well as other insulating part,s because of their superb electrical insulation capabilities.
- Consumer Goods: The exceptional durability of these materials allows for their use in the production of sporting goods, kitchen utensils, and even textile fibers.
- Industrial Applications: Their versatility and strength against wear make these materials applicable in the manufacture of bearings, conveyor belts, and even other types of machinery.
The versatility and efficiency of these applications in various settings are illustrated in the broad list of their uses.
How is nylon PA6 used in the automotive industry?
Because of its mechanical properties, lightweight, heat resistance, and other features, Nylon PA6 is essential to the automotive sector. It is used in constructing components like oil pans, engine covers, intake manifolds, and radiator tanks. The materials used in these applications must have strong temperature resistance, excellent strength-to-weight ratio, and optimum temperature resistance to metals.
Text cont Nylo PA6 is among the primary drivers in multitasking the overall power efficiency of the vehicles by drastically reducing the net weight of the components, leading to enhanced fuel efficiency and emissions control, which is the primary focus in the cutting-edge design of automobiles nowadays. For instance, replacing metal with nylon PA6 components in the market for intake manifolds results in intake manifolds that are up to 50 percent lighter in weight while outperforming competitors and maintaining durability. Beyond that, other qualities of nylon PA6, such as resistance to oil and coolant, guarantee that these materials are suitable for under-the-hood applications of vehicles and are even reliable in extreme scenarios.
The cross-sectional growth for the use of nylon PA6 in the automotive industry across the globe is on the rise in concordance with the shift of the automobile industry towards sustainability and the drive to make the automobile industry more noticeable. As per general consensus, lightweight polymers such as PA6 will comprise more than 35 percent of the global share in vehicle materials by 2030, This clearly indicates the escalating value such polymers present for advancement and innovation in automobile technology.
Why is nylon PA66 preferred for engineering plastic applications?
Nylon PA66 is exceptionally famous for its mechanical engineering applications due to the exceptional mechanical properties, thermal resistance, and durability it provides. Its remarkable tensile strength and stiffness bestow paws with prowess PA66 is suitable for strenuous conditions. PA66 boasts of a melting point of about 266°C which gives it thermal stability superiority over other variants of nylon. This enables it to endure high operating temperatures, making it suitable for industrial parts and automotive components that are heated.
Also, PA66 has good wear resistance as well as low friction, ensuring dependability for moving or repeatedly actuating parts. Its moisture absorption rate declines, comparatively reducing dimensional changes and augments performance stability across various climate conditions. Automotive, electrical,l and consumer goods industries have started utilizing PA66 for gears, housings, connectors, and bearings since it retains its mechanical strength under load. As noted in industry analyses, the global PA66 market is projected to rise considerably, estimated to touch $9 billion by 2027 due to its wide range of applications and efficiency in high-performance areas.
What role does glass fiber reinforcement play in PA6 and PA66?
The mechanical functioning of PA6 and PA66 is greatly improved with glass fiber reinforcement, making these materials ideal for demanding applications. Glass fibers augment the tensile strength, stiffness, and dimensional stability of PA6 and PA66 while also decreasing the materials’ creep susceptibility under constant stress. For instance, glass fiber reinforced PA66 has some configurations with tensile strengths exceeding 200 MPa while unreinforced versions typically range between 50-80 MPa.
Furthermore, the addition of glass fibers improve the thermal stability PA6 and PA66 allowing them to retain their shape and functionality at higher elevation, frequently up to 120-150°C, during continuous use. This is particularly favorable for components used in automotive under-the-hood regions, components used structurally in electronic devices, and industrial machinery.
Durability further improves as a result of enhanced resistance to wear and fatigue owing to glass fiber reinforcement. PA6 and PA66 with glass fibers demonstrate exceptional performance in applications involving moving parts, such as gears and bearings, where durability is critical. The market for glass fiber reinforced nylon compounds is anticipated to grow steadily, owing to the widespread use of these materials for lightweighting and sustainable material innovation in the automotive and aerospace industries.
How do PA6 and PA66 differ in terms of chemical resistance?
The difference in chemical resistance of PA6 and PA66 is primarily due to their molecular structures. Compared to PA6, PA66 has a greater resistance to hydrocarbons, oils, and greases. In contrast, PA6 exhibits superior resistance to acids and bases. Such variations make each of the materials more appropriate for particular environments of application, depending on the type of chemicals involved.
Which polyamide offers better chemical resistance?
While comparing both PA6 and PA66 with respect to the chemical resistance, the environment of application needs to be highlighted and assessed. It is well-known that PA66 is far superior to PA6 in resisting hydrocarbons, gasoline, diesel, oils, and grease, which propels it to be used within automotive components and industrial parts that are routinely subjected to such exposure. For instance, some studies show that PA66’s endurance to hydrocarbons at higher temperatures, unlike most plastics that become soft and pliable, poses no significant degradation, which depicts how robust it is in harsh chemical environments.
However, PA6 does a better job at withstanding acidic and basic environment,s which allows for utility in packaging, textiles, consumer goods, and other domains where cleansing agents and acidic solutions are commonplace. It is worth noting that while PA6 and PA66 share a remarkable array of similarities, their molecular differences imply drastic performance variability under chemical exposure; this becomes pertinent in deciding which material to use for which application.
How does the chemical resistance affect their applications?
The chemical resistance properties of PA6 and PA66 greatly influences their applicability in different use cases. Resistant to numerous hydrocarbons, as well as oils and greases, PA6 is highly favored in automotive and industrial applications. However, PA6 is slightly outperformed by PA66 in terms of the resistance against strong acids and bases. PA66 is significantly better than PA6 at withstanding harsher chemical environments, especially when subjected to high temperatures over prolonged periods, making it more suitable for demanding engineering applications, such as chemical processing equipment and industrial machinery.
Findings from studies conducted on materials’ performance indicate that PA66 retains approximately 70–80 percent of its tensile strength after enduring aggressive chemical exposure, while PA6 sustains around 60 percent, showcasing PA66’s edge for extreme environment usage. Moreover, PA6’s lower crystallinity, in relation to PA66, yields poorer chemical resistance, albeit enhancing processability and impact resistance in milder environments, such as consumer products or general-purpose packaging. The noticeable differences in chemical resistance of the two materials strongly suggest that the choice of these materials must be dictated by the requirements of the application.
What is the process for recycling PA6 and PA66?
Typically, the recycling of PA6 and PA66 incorporates two main processes:
- Mechanical Recycling: This involves collecting the materials, which requires both cleaning and shredding them into small pellets or flakes. These recovered pellets are reprocessed into new products, though they may degrade in quality with every cycle.
- Chemical Recycling: This entails polymers getting depolymerized to basic monomers which are then purified to be reassembled into either PA6 or PA66 of virgin material quality.
Though both techniques aid in waste minimization and reducing environmental effects, chemical recycling is often favored for its high-quality results.
What are the challenges in recycling PA6?
The recycling of PA6 (Polyamide 6) is its implementation on a broader scale is likely facing technical and economic challenges. One of the most severe challenges is the contamination of waste streams with impurities like other polymers, dyes, or additives, which compromises the quality of the recycled material. The composition of PA6 waste is often collected in a non-uniform manner, which incurs costly sorting and preprocessing procedures, adding to the expense and difficulty of recycling processes.
Furthermore, the mechanical recycling of the material results in a deterioration of its properties after a few cycles due to polymer chain scission. This makes it impossible for recycled PA6 to be used in high-performance applications. Alternatively, chemical recycling does allow for the polymers to be restored to their monomer state but requires considerable operational expenditure as well as sophisticated infrastructure due to the energy-intensive nature of the procedure.
Other market-based challenges come into play as well. Competition does allow for value to be created through less costly processes, meaning the demand for recycled materials won’t offset the expense of recycling when the price of virgin PA6 is sold at an effective deal. Data indicates that PA6 recycling remains unfeasible in many regions due to ineffective collection systems and a lack of economic incentives. Collectively, these issues assist in providing reasoning as to why recycling technology, waste management systems, and policy planning aimed at making PA6 recycling easier are required.
How is recycling PA66 different from PA6?
Recycling PA66 is markedly different from recycling PA6 due to their differences in chemical structure, physical properties, and other process requirements. PA66 is more difficult to recycle than PA6 due to its higher melting point and greater strength. The increased processing temperatures needed for PA66 also lead to more energy being used in recycling the material. Furthermore, the more severe material property degradation of recycled PA66 compared to PA6 limits its use in a greater number of applications than PA6 can.
Data shows that the mechanical PA66 recycling resulting from the initially advanced mechanical methods typically leads to the softened state and therefore lower mechanical strength and durability, rendering the recycled PA66 more suited for downcycling. High-performance applications would not be feasible with PA66 from downcycling, though. Increased possibility is seen in newer chemical recycling PA66 technologies and even some depolymerization techniques. Some recent research and pilot studies have showcased these techniques’ capabilities of deconstructing PA66 into its monomers, capable of being economically reformulated into a new grade of PA66.
Because of the intricate factors at play, PA66 has a low recycling rate in several regions. Some industries are adopting closed-loop systems and blending recycled PA66 with virgin material to improve performance and broaden the applicability of recycled content. Investment toward overcoming these challenges is necessary and should include advancing collection infrastructure, incentivizing PA66 recycling economically, and developing more effective technology for waste processing.
Reference Sources
-
Comparison of Nanocomposites Based on Nylon 6 and Nylon 661:
- Key Findings: PA6 nanocomposites exhibited superior mechanical properties compared to PA66, attributed to better exfoliation of organoclay in PA6. PA66 showed higher crystallinity but lower mechanical performance at high filler content.
- Methodology: Nanocomposites were prepared using twin-screw extrusion, and properties were analyzed using WAXD, TEM, and TGA.
-
Technological Approaches and Applications of PA6 and PA122:
- Key Findings: PA6 offers higher strength and thermal resistance, while PA12 provides better dimensional stability and lower moisture absorption. Applications span the automotive and aerospace industries.
- Methodology: Comprehensive review of additive manufacturing techniques like SLS and MJF, focusing on mechanical and thermal properties.
-
Fire Retardant Treatments for PA6 and PA663:
- Key Findings: Advances in halogen-free flame retardants improved the safety and environmental compatibility of PA6 and PA66. Challenges include balancing flame retardancy with mechanical performance.
- Methodology: Review of recent innovations in flame retardant formulations and their integration into textiles and composites.
- Top PA66 Plastic Pellets Suppliers in China
Frequently Asked Questions (FAQs)
Q: What is the main difference between PA6 and PA66 in terms of chemical composition?
A: The main difference between PA6 and PA66 lies in their chemical structure. PA6, also known as nylon 6, is produced through the ring-opening polymerization of caprolactam, while PA66 is formed by the polycondensation of hexamethylenediamine and adipic acid. This fundamental difference influences their physical properties and applications.
Q: How do the mechanical properties of PA6 and PA66 compare?
A: PA66 has a higher tensile strength and stiffness compared to PA6, making it suitable for applications requiring high mechanical strength. However, PA6 offers excellent mechanical properties and is known for its flexibility and impact resistance, which can be advantageous in certain applications.
Q: What are the differences in temperature resistance between PA6 and PA66?
A: PA66 generally exhibits better temperature resistance than PA6, with a higher melting point. This makes PA66 more suitable for applications that require exposure to higher temperatures, while PA6 is better suited for environments with moderate temperature requirements.
Q: How do PA6 and PA66 differ in terms of molding and processing?
A: Both PA6 and PA66 can be injection molded, but PA6 usually has better flow properties, making it easier to mold complex shapes. The mold temperature will affect the final properties of the molded product, with injection mold nylon PA66 requiring higher mold temperatures compared to PA6.
Q: What are the typical applications for PA6 and PA66?
A: PA6 is commonly used in applications such as automotive components, electrical housings, and consumer goods due to its excellent mechanical properties and flexibility. PA66, on the other hand, is widely used in industrial applications that demand higher mechanical strength and temperature resistance, such as gears, bearings, and structural components.
Q: Are there any cost differences between PA6 and PA66?
A: Generally, PA6 is less expensive than PA66 due to its simpler production process and lower material cost. This cost difference can be a significant factor when choosing between the two types of nylon for specific applications.
Q: How do PA6 and PA66 perform in terms of moisture absorption?
A: Both PA6 and PA66 are polyamides, which are hygroscopic and absorb moisture from the environment. However, PA6 tends to absorb more moisture than PA66, which can affect its dimensional stability and mechanical properties. This characteristic should be considered in applications where the material has been exposed to varying humidity levels.
Q: Can PA6 and PA66 be used interchangeably?
A: While PA6 and PA66 are both part of the nylon family and have some similar properties, they are not always interchangeable due to their differences in mechanical strength, temperature resistance, and moisture absorption. The selection between them should be based on the specific requirements of the application.
Q: How does the differentiation between PA6 and PA66 impact their environmental resistance?
A: PA66 generally offers better resistance to chemicals and environmental factors compared to PA6. This increased resistance makes PA66 preferable for applications exposed to harsh environments, although it comes at a higher cost than PA6.



